

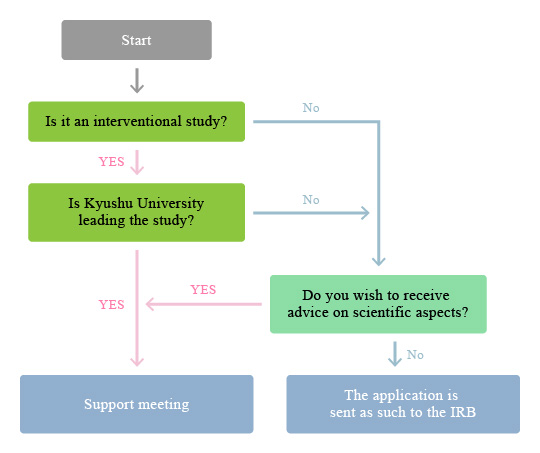
Download from the CCTR website the appropriate application form depending the type of study and prepare the application, study protocol and other documents necessary for the IRB examination.
It is preferable to have definite information on research funding, etc. at this stage.
≫Preparation of application for examination
http://aro.med.kyushu-u.ac.jp/research/template.html (in Japanese)
Submission of the application to CCTR, selection of specialists and fixing a suitable date for preliminary examination
Preliminary examination by specialists selected from the 100-member panel (1 hour)
New information added or the contents corrected as needed to make the application a superior research project proposal
Submission to the appropriate IRB for the main examination and approval by the IRB
Note: Please complete the registration with a Clinical Trial Registry before start of the study.
Study initiation, auditing and monitoring
All adverse events that occur must be notified
Support for analysis and preparation of research reports and scientific papers