


Note: This procedure is applicable for providing regenerative medical care through clinical research or treatment at the patient’s own expense. In case of a clinical trial, however, follow the procedure applicable for ordinary clinical trials in compliance with the Pharmaceutical and Medical Devices (PMD) Act.
Note: In principle, we do not accept Class 3 regenerative medical care projects from outside the Kyushu University for review.
Arrange for suitable compensation measures such as enrollment in a clinical research insurance scheme for regenerative medical care.
Any such insurance cover contract should be signed before the start of providing regenerative medical care, etc.
Window for insurance cover estimation and procedure for obtaining such cover (only for Kyushu University):
Sasahara, Strategic Planning Section, Kyushu University Hospital (extension: 5082)
Note: Those outside the Kyushu University may enquire about the insurance cover at their respective institution, or consider subscribing to a suitable insurance scheme like “Regenerative Medical Care Clinical Research Compensation Insurance Scheme” initiated by the Japanese Society for Regenerative Medicine.
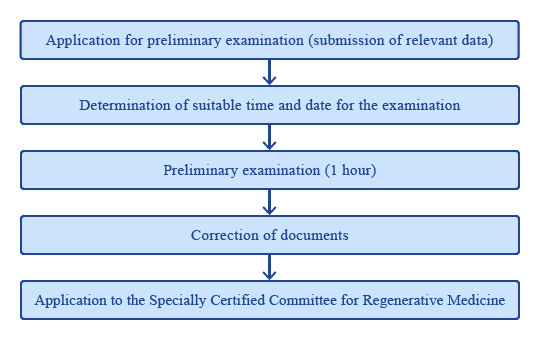
Note: What is preliminary examination?
Preliminary examination is an examination by experts from CCTR and the 100-member panel before the application is sent to the Specially Certified Committee for Regenerative Medicine. They offer general advice on preparation and implementation of the protocol (support for scientific rationality, biostatistics, and preparation of the informed consent form).
Download the following forms to prepare the documents
Submit one copy each of the following documents for preliminary examination.
⇒ Submit to: CCTR (Strategic Planning Section, 5 F Outpatient Building, Kyushu University Hospital)
TEL:092-642-5082 (Extension 3771)
E-mail:bynintei@jimu.kyushu-u.ac.jp
After deciding a suitable date and time, the preliminary examination is done in about 1 hour.
Any doubt or issue about details of the project is deliberated in the preliminary examination.
The documents for application to the Specially Certified Committee for Regenerative Medicine must be finalized on the basis of results of the preliminary examination.
Conflict of interest issues are examined in the preliminary examination. However, the Conflict of Interest Management Committee for Clinical Research is consulted if additional discussion is considered necessary.
| Within the University | Outside the University | |
|---|---|---|
| New examination | ¥237,000 | ¥387,000 |
| Re-examination | ¥142,000 | ¥219,000 |
| Report | - | ¥1,300 |